
Specialists in Delphi studies and sensitive data collection
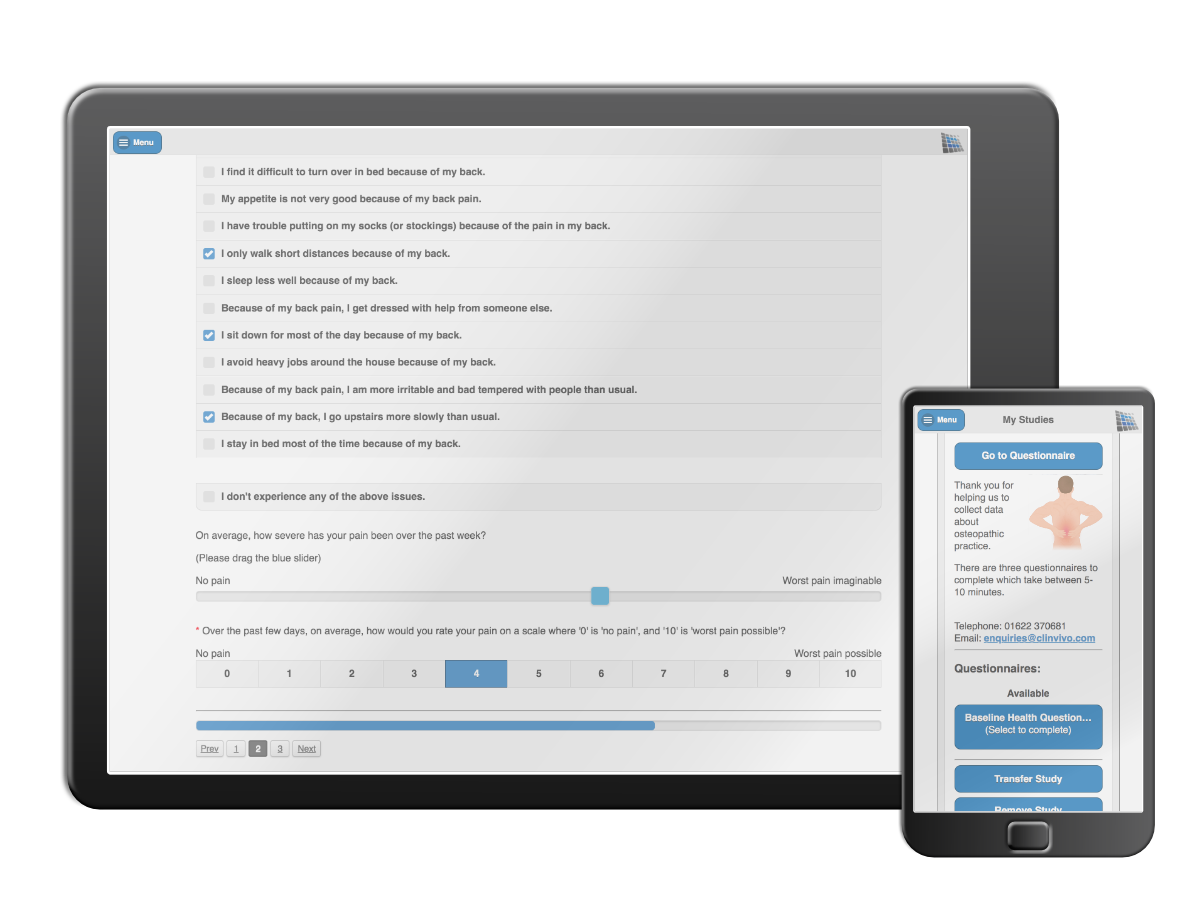
Data collection made easy
Our software and expertise in data capture helps academics to efficiently and securely obtain outcome data in trials, or get consensus in Delphi studies. No fiddly set-up work is needed - we get everything ready as it's needed and pilot with the academic team before going live.
Delphi studies
We help academics to design high-quality Delphi studies with low panel attrition. We increase research capacity by using our adaptable Delphi software to administer the study on behalf of teams, and we analyse responses and provide full inter-round and final reports.
EDC in trials and health studies
Our secure in-app and on-line managed solutions help academics and triallists collect data in health studies and trials, avoiding the need to pay for paper, postage, data entry, gaining higher response rates, while avoiding the need for any labour intensive set-up work or individual field encryption.
Routine data collection
Tried and tested methods to help professional bodies acquire feedback from stakeholder groups to ensure the quality of services delivered.
Products and Services

Contact
Chequers Barn, Chequers Hill, Bough Beech, Edenbridge, Kent, TN8 7PD



